Dr. Hekmat CONSULTING & Interim Management
Medical Devices - Regulatory Affairs & Quality Management System
https://www.healthcare.siemens.de/surgical-c-arms-and-navigation/mobile-c-arms
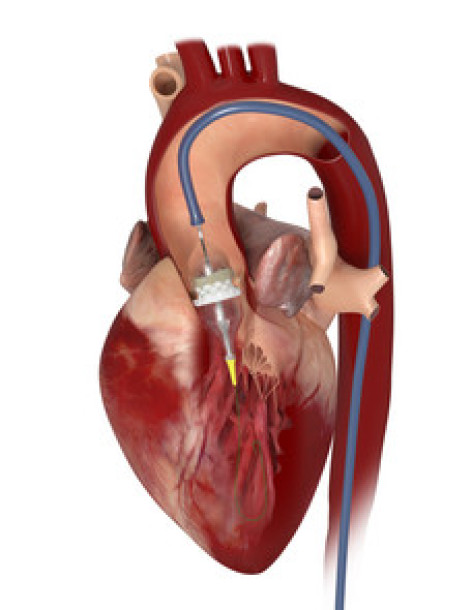
Source: aerztezeitung.de/medizin/krankheiten/herzkreislauf/article/898148/klappen-op-tavi-schneidet-besser-ab
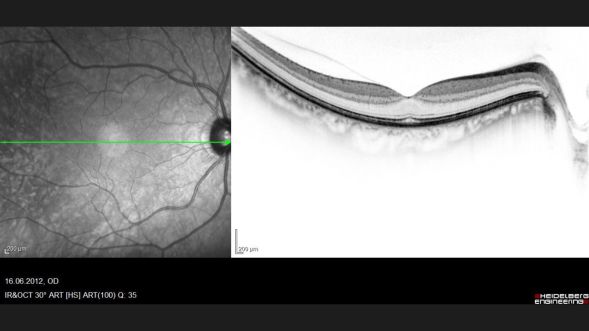
Optical coherence tomography (OCT), diagnostic ophthalmology, Dr. A. Hekmat 2012
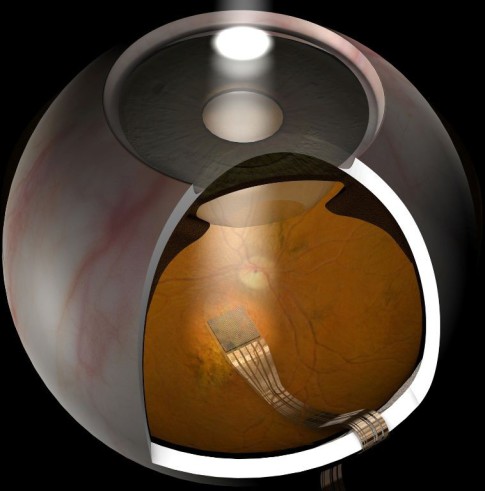
Sub-Retinal Implant (active neuro-implant), Retina Implant AG,
courtesy of Prof. Robert MacLaren, Oxford, UK
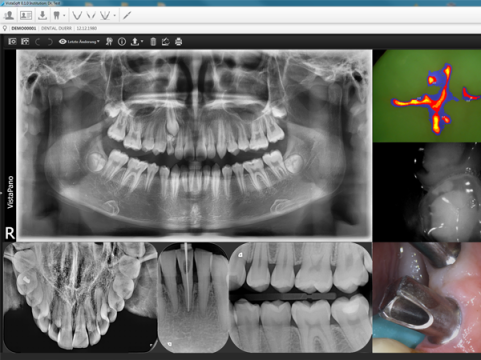
Source: Dürr Dental
Source: Dürr Dental
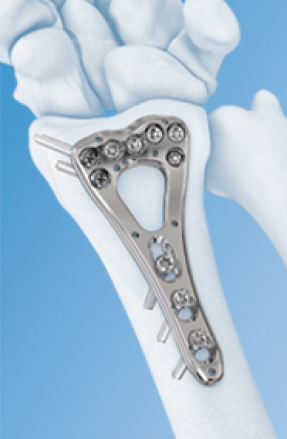
Source: DePuy Synthes
Source: wikipedia.org/wiki/Herz-Lungen-Maschine / heart-lung-machine